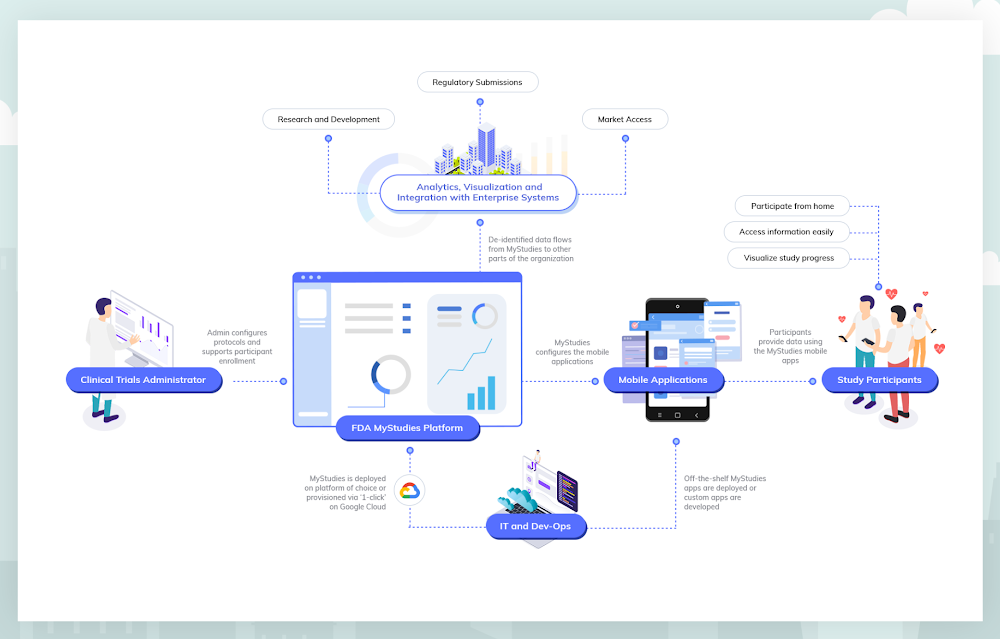
Google Cloud is committed to helping customers conduct life-saving research that results in new medications, devices and therapeutics by unlocking the knowledge hidden in real-world data. That’s why we’re supporting the goals of the U.S. Food & Drug Administration, by making the FDA’s open-source MyStudies platform available on Google Cloud Platform. By building on the platform developed by the FDA, we hope to stimulate an open ecosystem that will improve the ability of organizations to perform research that leads to better patient outcomes. This collaboration continues our long history of open-source work, and our commitment to producing easy-to-use tools that serve the healthcare and life sciences community.Because of the FDA’s focus on real-world evidence, drug and device organizations are increasingly looking to incorporate patient-generated data into regulatory submissions for new products and treatment indications. But until recently, there haven’t been mobile technologies or methodologies to help collect, store and submit this kind of data in a regulatory compliant manner. In order to address this gap, the FDA developed MyStudies, an open-source technology platform that supports drug, biologic and device organizations as they collect and report real-world data for regulatory submissions.Google Cloud is now working to expand the FDA’s MyStudies platform with built-in security and configurable privacy controls, and the ability for research organizations to automatically detect and protect personally identifying information. When an organization deploys FDA MyStudies on Google Cloud, a unique and logically isolated instance of the platform is created that only that organization and its delegates are authorized to access. These technologies will allow a research organization to select which of its researchers and clinicians are able to access what data, and to help optimize the use of that data as directed by participants. By leveraging Google Cloud as the underlying infrastructure for their FDA MyStudies deployments, organizations will have more safeguards in the ownership and management of data in their studies.Further, Google Cloud is providing sponsorship to bring Stanford University’s MyHeart Counts cardiovascular research study onto the FDA MyStudies platform, enabling this groundbreaking virtual clinical study to begin enrolling users of both Android and iOS devices. Since it launched as one of the initial iOS research applications, MyHeart Counts has enrolled more than 60,000 participants and driven significant understanding of the feasibility of conducting large-scale, smartphone-based clinical trials. Enabling patient-reported data with MyStudiesThe FDA relies on clinical trials and studies submitted by study sponsors to determine whether to approve, license or clear a drug, biologic or device for marketing in the United States. Historically, this information has been obtained almost exclusively through traditional clinical trials conducted under tightly controlled conditions. However, the increased digitalization of patient healthcare data may help to improve health with high-quality real-world evidence and more efficient clinical trials.The FDA has recognized this opportunity. For example, the agency’s Patient Engagement Advisory Committee is now helping assure the experiences of patients are included as part of the FDA’s deliberations on complex issues involving the regulation of medical devices. And, in 2017, the FDA Center for Devices and Radiological Health released a guidance document addressing real-world evidence generation for medical devices. The FDA has also released several draft Patient-Focused Drug Development guidance documents addressing how stakeholders can collect and submit patient experience data to support regulatory decision-making. Finally, in 2018, the FDA also released a Real-World Evidence Framework which details the agency’s efforts to evaluate real-world evidence for drugs and biologics as mandated by the 21st Century Cures Act.Originally launched as a publicly available resource in November of 2018, FDA’s MyStudies platform includes important features supporting patient accessibility and privacy. The patient-facing mobile application was built for Android using the open-source ResearchStack framework, and for iOS using Apple’s ResearchKit framework. By using these frameworks, developers can expand the capabilities of open-source mobile applications or create their own proprietary and branded applications. MyStudies mobile applications are configurable for different therapeutic areas and health outcomes through a web-based interface that reduces the need for custom software development. The overall platform has been designed to support auditing requirements for compliance with 21 CFR Part 11, allowing the platform to be used for trials under Investigational New Drug (IND) oversight.Study sponsors have already leveraged the FDA’s existing MyStudies platform to build branded and customized mobile applications to administer questionnaires that assess patient-reported outcomes, patient reports of prescription and over-the-counter medication use, trial medication diaries and other patient experience data. Supporting MyStudies on Google Cloud will make it even easier for new study sponsors to benefit from the MyStudies platform.New platform, new opportunitiesNow, Google Cloud is equipping the FDA’s MyStudies platform with an additional set of capabilities that reduce complexity and overhead, allowing pharma and medtech organizations to get up and running fast. For study designers who do not want to configure a compliant environment from scratch, a ‘click-to-deploy’ option will be available in the Google Cloud Marketplace later this year. When deploying FDA MyStudies on Google Cloud using this option, a private MyStudies instance is built from the open-source repository. That instance is then configured following best practices to operate with selected Google Cloud services. This allows research groups to establish their own, preconfigured instance of the FDA’s MyStudies platform in minutes.“Consistent with our obligations under the 21st Century Cures Act, FDA engages in public-private demonstration projects to advance the regulatory science around real-world evidence. The Patient Centered Outcomes Research Trust Fund investment that launched FDA MyStudies is a step toward this goal,” said David Martin, MD, associate director for Real-World Evidence Analytics, Office of Medical Policy, FDA Center for Drug Evaluation and Research. “FDA MyStudies is publicly available, but it requires professional expertise and time to progress from open-source resources to deployment of a new re-branded platform. As a company may do, Google Cloud is taking these resources and creating a click-to-deploy option linked to additional health data management and analytics.”Besides streamlined deployment of the open-source software, drug and device companies running FDA MyStudies on Google Cloud can benefit from integration with other Google Cloud offerings, such as managed services that support HIPAA compliance like the Healthcare API and our serverless data warehouse, BigQuery. More information about compliance on Google Cloud and an up-to-date list of products covered by our BAA can be found here.In addition to HIPAA compliance, Google Cloud can support customer compliance with CFR 21 Part 11 regulations when using Google Cloud in a prescribed manner to handle related data and workloads. While Google has a cloud technology stack that is ready for many CFR 21 Part 11 compliant workloads, the ultimate compliance determination depends on configuration choices made by the customer.MyHeart Counts + FDA MyStudies on Google CloudStanford University made mobile health history when it launched MyHeart Counts in 2015 as part of the inaugural cohort of iOS research applications. As an open enrollment study, any eligible individual who downloads the MyHeart Counts app may consent to participate in cardiovascular research. Once enrolled, participants are asked survey questions related to their health and physical activity. Participants may allow MyHeart Counts to collect physical activity data from their phone and other wearable devices. If participants are physically able, they will be asked to perform a 6-minute walk test, then enter information about risk factors and blood tests, which is used to determine a cardiovascular risk score.The current version of MyHeart Counts is only available on iOS devices. By using FDA MyStudies on Google Cloud, the Stanford researchers behind MyHeart Counts will conduct a multi-arm, randomized controlled trial that runs on both Android and iOS devices—the first of its kind. Additional improvements to the FDA MyStudies platform will allow researchers like those conducting MyHeart Counts to configure and deploy studies in days rather than months, without needing to develop any software.The study is being overseen by Professor Euan Ashley, MBChB, DPhil, professor of medicine, of genetics and of biomedical data science at Stanford. “In this digital era where everyone uses a smartphone, hosting a trial on an app lets us tap into a huge population. We are grateful for Google’s support because it enables us to expand our reach to include Android participants in addition to iOS, and incorporate an open-enrollment randomized controlled trial into a mobile application for the first time,” Prof. Ashley said.“MyHeart Counts and digital apps like it allow experts to connect directly to patients in a way that’s more immediate and more extensive, through direct, sensor-based measurement collection. Google Cloud’s support of these efforts not only helps researchers organize and deploy important research programs faster and more reliably, but ultimately will help patients and doctors notice health issues early, so they can address them sooner,” said Prof. Ashley.What’s next?In the spirit of our commitment to healthcare and open-source, Google Cloud will continue investing in MyStudies to bring general improvements to the platform, expand the number of supported assessments and enable integration with downstream analytics and visualization tools.Get in touch to learn more and be notified when FDA MyStudies becomes available on the Google Cloud Marketplace.Click to enlarge
Quelle: Google Cloud Platform
Published by